Hydrogen Inhalation Therapy and Its Role in Ovarian Cancer
November 18, 2022 2022-11-28 7:27Hydrogen Inhalation Therapy and Its Role in Ovarian Cancer

Hydrogen Inhalation Therapy and Its Role in Ovarian Cancer
Hydrogen Inhalation Therapy and Its Role in Ovarian Cancer
Introduction:
Worldwide, cancer is the leading cause of death, and its incidence continues to rise. There have been significant advances in basic and clinical cancer research over the past fifty years, resulting in a decline in mortality and incidence of certain cancers. Current cancer treatments, such as radiotherapy, chemotherapy, fine-needle aspiration, and surgery, can be a dual-edged sword as they can increase the number of circulating tumor cells and promote progression and spread of cancer (Neeman and Ben-Eliyahu, 2013, Karagiannis et al., 2017). Targeted precision therapeutic strategies based on molecular detection have not proven beneficial for the majority of cancer patients, with only 3-13% of patients having access to precision drugs. According to many studies, precision therapy induces proteome secretion in high amount, resulting in cancer metastasis. Therefore, alternative cancer therapies are necessary to avoid these challenges.
Since 2007, when Ohsawa and his colleagues published their startlingly effective therapeutic strategy results using molecular hydrogen on a rat model of cerebral infraction in Nature Medicine, it has been considered as a therapeutic and preventive medical gas. According to Ohsawa et al. hydrogen gas functions as a medicinal antioxidant by decreasing harmful oxygen radicals selectively. Acute oxidative stress generated by ischemia-reperfusion or inflammation produces severe tissue damage, and chronic oxidative stress is recognized as one of the major causes of prevalent diseases like, cancer (Ohsawa et al., 2007). Advanced cancer treatment presents a serious challenge, demanding the development of novel concepts and methods.
Molecular Hydrogen Basics:
Hydrogen gas exhibits antioxidant and anti-inflammatory properties that may be utilized to combat cancer, the occurrence and advancement of which are closely linked to peroxidation and inflammation. The hydrogen gas is a moderate yet powerful antioxidant. It is the most abundant molecule in cosmos, comprising almost 75% of the universe’s mass. Hydrogen is a colorless, tasteless, and odorless diatomic gas with chemical formula H2 (Huang et al., 2010). Hydrogen gas inhalation is a basic treatment approach. It can be inhaled using ventilator circuit, facemask, or nasal cannula to administer hydrogen gas. Since inhaled hydrogen has a quicker onset of action, it shows effective protection against acute oxidative stress (Ohta, 2011).
Hydrogen Inhalation Role in Cancer Management:
Hydrogen inhaled by patients with advanced cancer can improve the quality of life and inhibit cancer progression. As a technique for clinical rehabilitation of patients with advanced cancer, hydrogen inhalation is a simple, low cost treatment with few adverse effects that when inhaled the molecules get into lungs, and directly into bloodstream where they reach each and every cell quickly (Xu et al., 2019).
Reactive oxygen species (ROS) are generated inside the body as a byproduct of oxidative phosphorylation in the energy metabolism of all aerobic organisms. When excessive ROS are created or endogenous antioxidant capacity is depleted, indiscriminate oxidation induces detrimental effects, leading to “oxidative stress”. A researcher explained in his work that OH free radicle is powerful enough to react with even inert H2, whereas O2.-, H2O2, and NO free radicles are insufficient to react with H2. In other words, hydrogen gas is mild enough to either interfere with metabolic redox processes or ROS involved in cellular signaling. (Ohta, 2014). Furthermore, it has been discovered that the biological and antioxidant benefits of H2 persists even after hydrogen gas has been eliminated from the body, especially at low concentrations, indicating that the process may have more to do with the modulation of antioxidant signaling than with the actual scavenging of free radicals (Dixon, Tang and Zhang, 2013). The nuclear translocation of nuclear factor erythroid-2 related factor 2 (Nrf2) may control the expression of genes involved in the oxidative stress management (Tonelli, Chio and Tuveson, 2018).
H2 gas decreased inflammation in animal models induced by Concanavalin A (Kajiya et al., 2009), dextran sodium sulfate (Kajiya et al., 2009), and lipopolysaccharide (LPS) (Chen et al., 2013). H2 gas, H2-saline, H2-water reduced the levels of pro-inflammatory cytokines to minimize inflammation. H2 has benefits against oxidative stress in addition to anti-inflammatory and anti-allergic properties. H2 regulates expression of several genes and phosphorylation of various proteins (Ohta, 2011). Despite numerous inaccuracies, selective free radical and inflammation scavenging ability are still widely acknowledged mechanism of H2. Pre-inhalation of H2 could prevent caerulein-induced acute pancreatitis in mice by reducing the early stage inflammation and oxidative stress, based on the evidence of a study (Li et al., 2021).
In the adaptive immune system, Xi et al. study discovered comparable outcomes, which were primarily caused by a rise in the number of tired and senescent T cell subsets as well as a drop in the proportion of functioning subsets. Additionally, they discovered proof of T helper cell type 1 (Th1) and follicular Th cells’ limited capacity for cytokine release. They found that there was a decreased proportion of normal killer T cells (NKT), activated NK and killer NK cells, and an insufficient content of V2 subsets among T cells in the detection of innate immune response. As a result, it is very likely that both the innate and adaptive immune systems work in joint contribution to advance the cancer progression. Their study provides the first concrete proof that two weeks of hydrogen inhalation can considerably slow down the process pf aging as well as innate and adaptive immunity (Xu et al., 2020).
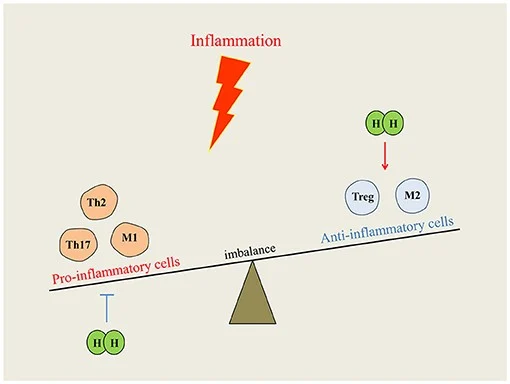
Figure: Hydrogen’s ability to modulate the immune system. Immune homeostasis is upset when inflammation takes place because of dis-organized immune cells. By downregulating pro-inflammatory cells or upregulating anti-inflammatory cells, hydrogen intervention could reduce inflammation and restore the equilibrium. (Tian et al., 2021).
Along with focusing on H2 neutralizing oxidative stress and immune response betterment, the mechanism prior to electron transport chain, which is the initial stage of mitochondrial oxidative stress, were also studied by researchers. Since they generate 90% of the cell’s energy in the form of ATP, mitochondria are frequently referred as the powerhouses of the cell. The production of ROS via forward and reverse electron transfer is accompanied by this mechanism, which depends on oxidative phosphorylation (Annesley and Fisher, 2019). Hydrogen gas is thought to have the capacity to restore cellular dysfunction by reducing uncontrolled electron leakage from electron transport chain, which improves mitochondrial function.
Genes or Signaling Pathways Over-expressed in Ovarian Cancer
For the earlier detection of epithelial ovarian cancer, MUC16 is a key tumor marker. It is frequently employed in clinical settings. According to a study, the serum MUC16 levels of 80% of ovarian cancer patients were raised. Additionally, The Cancer Genome Atlas (TCGA) effort focused on ovarian cancer discovered that the abnormal increase of tumor marker MUC16 in ovarian cancer plays a significant role in the onset and progression of ovarian cancer. Both the amplification of the gene that encodes MUC16 and the expression of MUC16 messenger RNA are strongly linked to the poor prognosis of ovarian cancer patients, according to the research (Rao et al., 2015).
Acetaldehyde dehydrogenases (ALDH) have been discovered to be crucial in the metabolism and carcinogenesis of tumor cells in recent years. ALDH1 is one of them and has a strong connection to tumor cell stemness. Acetaldehyde dehydrogenases A1(ALDH1A1) is one of the crucial members of the superfamily among the several ALDH1 families. The physiological role of ALDH1A1 is to take part in retinoic acid metabolism and to encourage the activation of the retinoic acid metabolic pathway. The function and relevance of this molecule in malignant tumors have garnered scholarly interest recently. According to studies, ALDH1A1 is abundantly expressed in ovarian cancer cells and contributes to the disease’s resistance to Chemoradiotherapy, which causes it to recur and spread (Condello et al., 2014).
According to Bodnar et al.21, activating the Wnt/-catenin signaling pathway could encourage the development and development of ovarian carcinoma cells while inhibiting apoptosis. The Wnt/-catenin signaling cascade involves numerous layers of negative modulators. Using the Cytoscape technique, the DEGs that are closely linked to the Wnt/-catenin signal transduction pathway in ovarian cancer were identified, with SFRP1, BAMBI, and WNT2B downregulated and SOX17, FZD10, and MMP7 expression elevated (Bodnar et al., 2014).
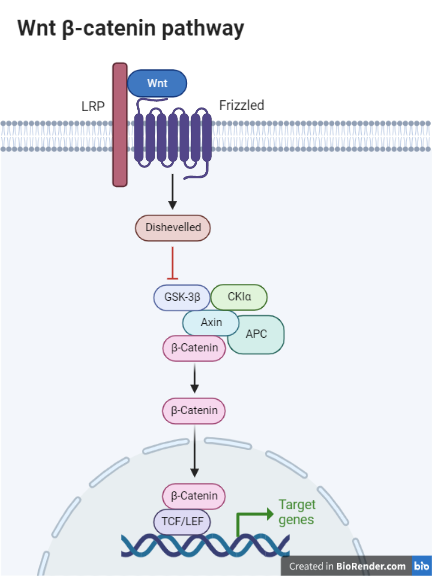
Illustration Created with BioRender.com
According to a different study, ALDH1A1 is -catenin’s target molecule, and -catenin knockdown can impair ovarian cancer cell viability, tumor growth, and metastasis. Therefore, creating ALDH1A1 inhibitors with more specificity could improve the efficacy of chemotherapy in treating ovarian cancer.
Hydrogen Inhalation Impact on Controlling Ovarian Cancer
Ovarian cancer has an insidious onset and no early warning symptoms, making it the fifth most incidence of cancer-related death in women (Karnezis et al., 2016). On the basis of biological origin, ovarian cancer can be roughly divided into three major categories, including epithelial cells (90%), sex-cord stromal cells (7%) and germ cells (3–7%) (Shanmugasundaram et al., 2015). Chemotherapy is frequently used in conjunction with significant surgery to treat ovarian cancer. Although Taxol and platinum-based chemotherapy produce promising outcomes, acquired resistance and unfavorable side effects pose significant obstacles to the successful treatment of ovarian cancer. The overall cure rate for ovarian cancer has stayed at 30% for the past 20 years, despite ongoing research into treatments (Bast, Hennessy and Mills, 2009). Exploring harmless, anticancer compounds is therefore crucial for the ovarian cancer treatment.
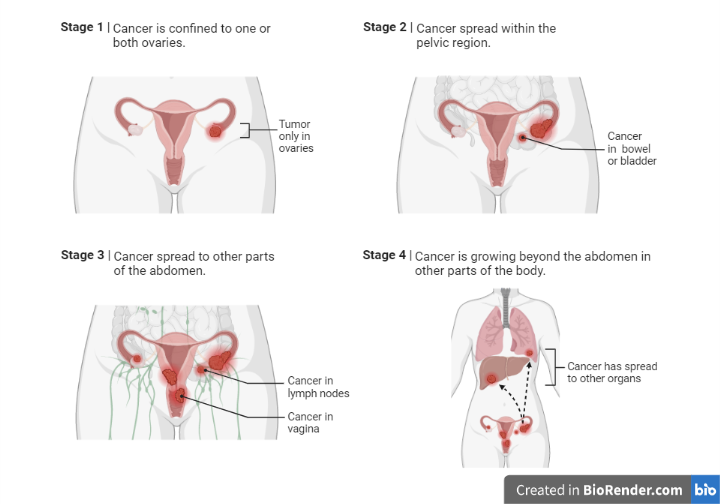
Figure: Staging and spread of ovarian cancer in women. Illustration Created with BioRender.com
Most studies on the biological effects of molecular hydrogen have concentrated on the inhalation of low concentrations of hydrogen gas (1-4%) or normobaric hydrogen since Ohsawa et al ‘s report. Only a few studies have been published, however hyperbaric hydrogen or high concentrations of hydrogen may be beneficial for some conditions. According to a study done by (Shang et al., 2018), breathing in gas that contains 66.7% hydrogen can have an anti-tumor effect on ovarian cancer. The dose-effect connection of hydrogen needs to be further researched, but these data suggest that greater concentrations of hydrogen may be more beneficial in the treatment of cancer.
Supporting Evidence of H2 Inhalation Therapy:
A 72-year-old woman with advanced gallbladder poorly differentiated adenocarcinoma was diagnosed with stage IV cancer. The patient then treated with hydrogen gas therapy, inhaling hydrogen through a nasal cannula. Within 1 month after treatment, gallbladder and liver tumors enlarged, CA19-9, Alpha Fetoprotein (AFP), and Carcinoembryonic Antigen (CEA) were all elevated, but patient felt better, peripheral red blood cells, hemoglobin and albumin were all increased. The tumor markers completely fell to normal range, and CT scan results showed greater than 50% reduction in gallbladder tumors (Kecheng X et al., 2019).
Another clinical trial based of 52 patients’ hydrogen gas inhalation therapy showed prolonged overall survival and progression-free survival in patients with stage IV colorectal cancer, and the mechanism led to recovery of CD8+ T cells (Akagi and Baba, 2018). Xu et al. published a study based on 82 cancer patients of lung, liver, and pancreas, in which they administered H2 gas inhalation in patients with stage III or IV. Results showed complete and partial remission, with a disease control rate of 57.5% (Xu et al., 2019).
In a research conducted by Akagi and Baba in 2020, on 42 lung cancer patients who were subjected to hydrogen gas inhalation therapy for 60 months and they showed better survival. Overall survival was prolonged when H2 gas and Nivolumab were combined, and the main factor involved in this, was the elevated level of coenzyme Q10 (Akagi and Baba, 2020). Inhaling H2 gas had a tumor reducing impact, according to a case study of a patient whose primary tumor was lung cancer which metastasized was to brain (Chen et al., 2019).
In a clinical trial, 48 lung cancer patients were provided with hydrogen gas inhalation therapy. 10 individuals received hydrogen gas alone and remaining in conjunction with anticancer medications. This treatment prolonged the progression free survival in individuals with lung cancer (Xu et al., 2020).
Conclusion:
To achieve therapeutic efficacy, inhaled hydrogen must be administered at sufficiently high concentrations to permit quick tissue penetration and for long enough to induce a dose-accumulation effect. Hydrogen gas can be used to minimize the cancer progression quite effectively.
References:
- Akagi, J. and Baba, H. (2018). Hydrogen gas restores exhausted CD8+ Tcells in patients with advanced colorectal cancer to improve prognosis. Oncology Reports. [online] doi:10.3892/or.2018.6841.
- Akagi, J. and Baba, H. (2020). Hydrogen gas activates coenzyme Q10 to restore exhausted CD8+ T cells, especially PD‑1+Tim3+terminal CD8+ T cells, leading to better nivolumab outcomes in patients with lung cancer. Oncology Letters, [online] 20(5), pp.1–1. doi:10.3892/ol.2020.12121.
- Annesley, S.J. and Fisher, P.R. (2019). Mitochondria in Health and Disease. Cells, [online] 8(7), p.680. doi:10.3390/cells8070680.
- Bast, R.C., Hennessy, B. and Mills, G.B. (2009). The biology of ovarian cancer: new opportunities for translation. Nature Reviews Cancer, [online] 9(6), pp.415–428. doi:10.1038/nrc2644.
- Bodnar, L., Stanczak, A., Cierniak, S., Smoter, M., Cichowicz, M., Kozlowski, W., Szczylik, C., Wieczorek, M. and Lamparska-Przybysz, M. (2014). Wnt/β-catenin pathway as a potential prognostic and predictive marker in patients with advanced ovarian cancer. Journal of Ovarian Research, [online] 7(1), p.16. doi:10.1186/1757-2215-7-16.
- Chen, J., Mu, F., Lu, T., Du, D. and Xu, K. (2019). Brain Metastases Completely Disappear in Non-Small Cell Lung Cancer Using Hydrogen Gas Inhalation: A Case Report. OncoTargets and Therapy, [online] Volume 12, pp.11145–11151. doi:10.2147/ott.s235195.
- Chen, H.-G., Xie, K.-L., Han, H.-Z., Wang, W.-N., Liu, D.-Q., Wang, G.-L. and Yu, Y.-H. (2013). Heme oxygenase-1 mediates the anti-inflammatory effect of molecular hydrogen in LPS-stimulated RAW 264.7 macrophages. International Journal of Surgery, [online] 11(10), pp.1060–1066. doi:10.1016/j.ijsu.2013.10.007.
- Condello, S., Morgan, C.A., Nagdas, S., Cao, L., Turek, J., Hurley, T.D. and Matei, D. (2014). β-Catenin-regulated ALDH1A1 is a target in ovarian cancer spheroids. Oncogene, [online] 34(18), pp.2297–2308. doi:10.1038/onc.2014.178.
- Dixon, B.J., Tang, J. and Zhang, J.H. (2013). The evolution of molecular hydrogen: a noteworthy potential therapy with clinical significance. Medical Gas Research, [online] 3(1), p.10. doi:10.1186/2045-9912-3-10.
- Huang, C.-S., Kawamura, T., Toyoda, Y. and Nakao, A. (2010). Recent advances in hydrogen research as a therapeutic medical gas. Free Radical Research, [online] 44(9), pp.971–982. doi:10.3109/10715762.2010.500328.
- Karnezis, A.N., Cho, K.R., Gilks, C.B., Pearce, C.L. and Huntsman, D.G. (2016). The disparate origins of ovarian cancers: pathogenesis and prevention strategies. Nature Reviews Cancer, [online] 17(1), pp.65–74. doi:10.1038/nrc.2016.113.
- Karagiannis, G.S., Pastoriza, J.M., Wang, Y., Harney, A.S., Entenberg, D., Pignatelli, J., Sharma, V.P., Xue, E.A., Cheng, E., D’Alfonso, T.M., Jones, J.G., Anampa, J., Rohan, T.E., Sparano, J.A., Condeelis, J.S. and Oktay, M.H. (2017). Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Science Translational Medicine, [online] 9(397). doi:10.1126/scitranslmed.aan0026.
- Kajiya, M., Sato, K., Silva, M.J.B., Ouhara, K., Do, P.M., Shanmugam, K.T. and Kawai, T. (2009). Hydrogen from intestinal bacteria is protective for Concanavalin A-induced hepatitis. Biochemical and Biophysical Research Communications, [online] 386(2), pp.316–321. doi:10.1016/j.bbrc.2009.06.024.
- Kajiya, M., Silva, M.J.B., Sato, K., Ouhara, K. and Kawai, T. (2009). Hydrogen mediates suppression of colon inflammation induced by dextran sodium sulfate. Biochemical and Biophysical Research Communications, [online] 386(1), pp.11–15. doi:10.1016/j.bbrc.2009.05.117.
- Kecheng X, Jibing C, Tianyu L and Youyong L (2019). Hydrogen Gas Inhalation for Treatment of Advanced Carcinoma. [online] Clinics in Oncology Research. Available at: https://austinpublishinggroup.com/clinics-in-oncology-research/fulltext/cor-v2-id1008.php
- Li, K., Yin, H., Duan, Y., Lai, P., Cai, Y. and Wei, Y. (2021). Pre-inhalation of hydrogen-rich gases protect against caerulein-induced mouse acute pancreatitis while enhance the pancreatic Hsp60 protein expression. BMC Gastroenterology, [online] 21(1). doi:10.1186/s12876-021-01640-9.
- Neeman, E. and Ben-Eliyahu, S. (2013). Surgery and stress promote cancer metastasis: New outlooks on perioperative mediating mechanisms and immune involvement. Brain, Behavior, and Immunity, [online] 30, pp.S32–S40. doi:10.1016/j.bbi.2012.03.006.
- Ohsawa, I., Ishikawa, M., Takahashi, K., Watanabe, M., Nishimaki, K., Yamagata, K., Katsura, K., Katayama, Y., Asoh, S. and Ohta, S. (2007). Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nature Medicine, [online] 13(6), pp.688–694. doi:10.1038/nm1577.
- Ohta, S. (2011). Recent Progress Toward Hydrogen Medicine: Potential of Molecular Hydrogen for Preventive and Therapeutic Applications. Current Pharmaceutical Design, [online] 17(22), pp.2241–2252. doi:10.2174/138161211797052664.
- Ohta, S. (2014). Molecular hydrogen as a preventive and therapeutic medical gas: initiation, development and potential of hydrogen medicine. Pharmacology & Therapeutics, [online] 144(1), pp.1–11. doi:10.1016/j.pharmthera.2014.04.006.
- Rao, T.D., Tian, H., Ma, X., Yan, X., Thapi, S., Schultz, N., Rosales, N., Monette, S., Wang, A., Hyman, D.M., Levine, D.A., Solit, D. and Spriggs, D.R. (2015). Expression of the Carboxy-Terminal Portion of MUC16/CA125 Induces Transformation and Tumor Invasion. PLOS ONE, [online] 10(5), p.e0126633. doi:10.1371/journal.pone.0126633.
- Shang, L., Xie, F., Li, J., Zhang, Y., Liu, M., Zhao, P., Ma, X. and Lebaron, T.W. (2018). Therapeutic potential of molecular hydrogen in ovarian cancer. Translational Cancer Research, [online] 7(4), pp.988–995. doi:10.21037/tcr.2018.07.09.
- Shanmugasundaram, G., Sundaramoorthy, E., Sudalaiandi, S., Kondaveeti, S.S., Johnson, T., Swaminathan, R. and Ramesh, A. (2015). Double Pathology: Malignant Epithelial Ovarian Tumor and Germ Cell Tumor (Choriocarcinoma), a Rare Coexistence. World Journal of Oncology, [online] 6(4), pp.421–425. doi:10.14740/wjon848w.
- Tian, Y., Zhang, Y., Wang, Y., Chen, Y., Fan, W., Zhou, J., Qiao, J. and Wei, Y. (2021). Hydrogen, a Novel Therapeutic Molecule, Regulates Oxidative Stress, Inflammation, and Apoptosis. Frontiers in Physiology, [online] 12. doi:10.3389/fphys.2021.789507.
- Tonelli, C., Chio, I.I.C. and Tuveson, D.A. (2018). Transcriptional Regulation by Nrf2. Antioxidants & Redox Signaling, [online] 29(17), pp.1727–1745. doi:10.1089/ars.2017.7342.
- Xu, K.-C., Chen, J.-B., Kong, X.-F., Lv, Y.-Y., Qin, S.-C., Sun, X.-J., Mu, F. and Lu, T.-Y. (2019). ‘Real world survey’ of hydrogen-controlled cancer: a follow-up report of 82 advanced cancer patients. Medical Gas Research, [online] 9(3), p.115. doi:10.4103/2045-9912.266985.
- Xu, K.-C., Chen, J.-B., Kong, X.-F., Mu, F., Lu, T.-Y. and Lu, Y.-Y. (2020). Hydrogen therapy can be used to control tumor progression and alleviate the adverse events of medications in patients with advanced non-small cell lung cancer. Medical Gas Research, [online] 10(2), p.75. doi:10.4103/2045-9912.285560.
- Xu, K.-C., Chen, J.-B., Kong, X.-F., Qian, W., Mu, F., Lu, T.-Y. and Lu, Y.-Y. (2020). Two weeks of hydrogen inhalation can significantly reverse adaptive and innate immune system senescence patients with advanced non-small cell lung cancer: a self-controlled study. Medical Gas Research, [online] 10(4), p.149. doi:10.4103/2045-9912.304221.

