Hydrogen Inhalation Therapy and Its Role as a Neuroprotective Agent
November 18, 2022 2022-11-28 7:27Hydrogen Inhalation Therapy and Its Role as a Neuroprotective Agent

Hydrogen Inhalation Therapy and Its Role as a Neuroprotective Agent
Table of Contents
Hydrogen Inhalation Therapy and Its Role as a Neuroprotective Agent
Background:
Neuro-inflammation is recognized in inducing neurology related problems, including those resulting from neurodegenerative-diseases, brain damage, and other diorders (Emerit, Edeas and Bricaire, 2004).
Neurological complications after surgery can range from a heavy stroke to a mild form of cognitive failure. Patients undergoing cardiac or vascular surgery, who are regarded to be at a higher risk for worse neurologic results, have been the focus of a large amount of work to improve outcomes. This effort has been focused on improving neurologic outcomes. Because there is a dearth of compelling clinical evidence, there are no established guidelines despite the fact that preclinical research has shown some encouraging results. The practice of neuroprotection can be classified into two primary categories: passive and active. Passive neuroprotection is defined as the avoidance of neurotoxic causes, whereas active neuroprotection involves the implementation of neuroprotective therapies.
Neuro-protective medicines are drugs that can change the metabolic events following the beginning of the issues like ischemia, and so have the ability to minimize damage due to stroke. Not a single chemical medicine has demonstrated apparent beneficial effects among these therapies. Many of the agents like blockers of calcium channel and some antagonists have theoretical or experimental promise, but none has demonstrated clinical effectiveness.
Effectiveness of H2 at the Molecular Level:
H2 is currently acknowledged as a gas with medicinal positive efficacy on several disorders, comprising neurological, metabolic, and inflammatory diseases. The neutral qualities of therapeutic H2 are believed to enhance the passive diffusion of H2 itself throughout the body soon after the supplying and to make sure a favorable safe profile as it is an inert gas and it does not disrupt any enzymatic processes in body.
Hydrogen gas inhalation is now considered a basic medicinal technique. Hydrogen gas may be inbreathed by means of a cannula, facemask, or even a ventilation machine. Due to the quick action of hydrogen gas, it could be an ideal situation to combat stress caused by oxidation. Specifically, the inhalation of gas has no effect on blood-pressure (Ohsawa et al., 2007).
In a study, group of scientists demonstrated that in approximately 20 minutes, the amount of hydrogen gas in blood of artery and veins achieved a plateau of 10–20 mM and had no effect on physiological markers, indicating no adverse consequences.
Multiple pieces of evidence suggest that hydrogen lowers reactive oxygen species that are highly reactive in the area of chronic illness. Both inhaling H2 gas and injecting HS are capable of delivering detectable levels of H2 to the brain.
Mechanism of Action of H2 as a Potential Medicine:
The precise chemical pathways behind the outcomes of lesser dose of hydrogen are still unknown. Multiple signal transduction pathways can be modulated by H2, although its basic molecular targets are unknown. Examining overlapped signaling molecules would aid in mapping cross-talk between important pathways. For a comprehensive explanation of roles of hydrogen gas in biological processes, and the molecular actions must be cleared. Proposed important mechanisms of action are shown in the illustration 1 below:
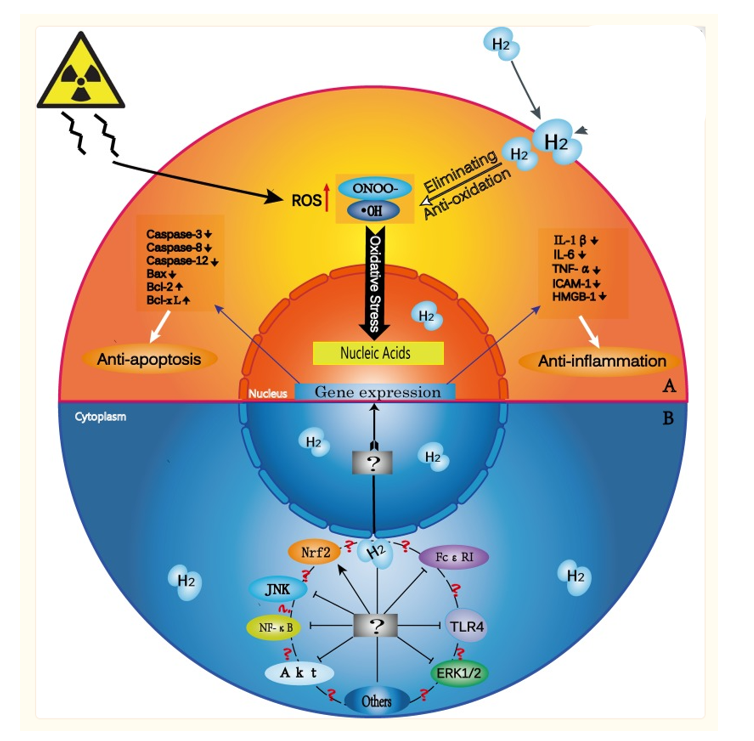
Illustration 1. Potential mechanisms of Hydrogen gas at molecular level.
(Ge et al., 2017)
Using a specialized electrode, the diffusion of hydrogen gas within different tissues could be tracked. For instance, the H2 content within the rat myocardial has been measured. In order to assess the diffusion of hydrogen into ischemic myocardial region following coronary artery closure, the sensors were placed in the “at hazard” zone for an infarct.. Even with coronary artery closure, H2 content was elevated due to diffusion (Hayashida et al., 2008).
Hydrogen Inhalation Against Ischemia
Ischemia poses a unique threat to the brain. It takes approximately 40 minutes of ischemia to destroy kidney or heart cells, yet just 5 minutes to kill susceptible neurons in numerous brain locations. The high metabolic rate of brain tissue contributes to its heightened susceptibility to ischemia injury. Although the human brain comprises just about 2.5 percent of the overall of body mass, it is responsible for 25 percent of metabolism, an energy expenditure that is three-and-a-half times that of other primate brains. Moreover, central neurons rely almost exclusively on glucose as an energy substrate, and brain glucose and glycogen stores are limited. In the past 15 years, however, evidence has accumulated suggesting that energetics considerations and energy substrate limitations are not the primary explanation of the brain’s increased sensitivity to ischemia. Under cerebral ischemia conditions, it appears that the innate cell-cell and subcellular signaling pathways of the brain, which are generally involved in information processing, become detrimental. This accelerates energy depletion and exacerbates the final steps that lead to ischemia cell death in all tissues. These pathways include free radical production, activation of enzymes for catabolism, breakdown of membranes, initiation of apoptosis, and the initiation of inflammation.
Annually, around 7 hundred thousand Americans experience a stroke, of which 600,000 are first-time occurrences. (Benjamin et al., 2018). Significant progress has been achieved in treating strokes due to acute ischemia, which now involves medicinal recanalization of occluded artery (Furie and Jayaraman, 2018). Despite this, stroke remains a primary reason for fatality. An approximate of 7 million Americans aged 20 years or older have experienced an attack of ischemia (Benjamin et al., 2018).
It has been shown that restoring blood flow does not terminate cellular harm. Despite the fact that neuroprotection studies on animal models of stroke have yielded promising outcomes, human therapies are unable to uncover any suitable molecule. Because of the complex nature of pathogenic reactions, cascades of apoptosis and cytotoxicity are unstoppable using a single molecule a single molecule. In addition, reduced distribution of the neuroprotective agent within the area of penumbra may diminish the impact. Therefore, a successful medicine must be readily accessible, diffuse swiftly, work on several linked pathogenic processes, and show minimal bad impacts on health.
Interest in studying hydrogen’s neuroprotective effects has increased lately. In contrast to its effects on oxygen free radical, hydrogen peroxide, and nitric oxide, recent studies show that H2 preferentially reduces the extremely dangerous ROS, OH and peroxynitrite. And H2 gas inhalation greatly lessens ischemic-reperfusion injury (IRI) in the parts of brain by combating stress caused by oxidation (Ohsawa et al., 2007). In subsequent research (Hayashida et al., 2008), hydrogen’s therapeutic effects on the heart IRI as well as liver IRI was demonstrated (Fukuda et al., 2007). The outcomes of these research studies poses that hydrogen can be a better effective anti-oxidant treatment than the solutions now available. Because H2 diffuses fast through cell membranes, it can access harmful ROS and interact with them. Since 2007, numerous studies (Ohta, 2014; Ichihara et al., 2015) have demonstrated the therapeutic efficacy of H2 against oxidative damage (Ohta, 2014; Ichihara as al., 2015). Numerous studies have shown that hydrogen gas minimizes the inflammation in animal models of disorders due to LPS (Itoh et al., 2011) and others (Ohta, 2014), with a decrease in quantity of certain cytokines.
Suggested H2 Delivery System:
Numerous ways exist for administering or ingesting H2 into the body. Inhaling hydrogen gas, ingesting water with dissolved hydrogen (HW), and injecting hydrogen added saline are the three general categories (HS). The doses and style of administrating hydrogen differs based according to the disease (Ichihara et al., 2015).
Since the first study by Ohsawa et al. in 2007, the inhalation of hydrogen has emerged as the technique that is the most widely used & the easiest to understand (Ichihara et al., 2015). H2 inhaled into the lungs distributes through passive diffusion into the alveoli and is transported through blood in the whole body. Nevertheless, the administration route can be difficult and quite dangerous due to the explosive nature of H2 gas over a concentration of 4% in air. Consequently, the concentration of H2 in a mixture of gases is typically kept between 1% and 4%.
(Masumi Iketani and Ikuroh Ohsawa, 2022)
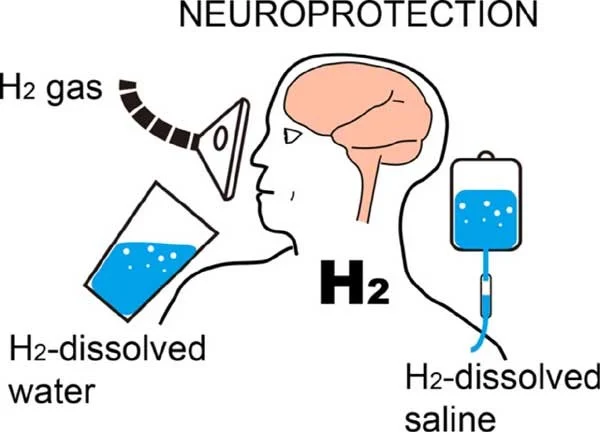
Injecting hydrogen dissolved saline is also risk-free, outside the possibility of any explosive outcome, and permits application of HS directly to the area that are affected. Intraperitoneal injections of HS have exhibited similar effects against IRI in the regions of brain as H2 inhalation, despite the invasive nature of the technique (Liu et al., 2011).
In addition, the use of bacteria that produce hydrogen in the digestive tract could be beneficial. For example, ingesting lactulose significantly increases the amount of hydrogen gas produced by gastrointestinal bacteria (Zhai et al., 2013).
H2 Inhalation Therapy for Infracted Brain Tissue as a Neuroprotective Agent:
The brain infarction is a pathological process that produces a region of necrotic brain tissue (cerebral infarct). It is caused by interrupted blood supply (ischemia) and restricted oxygen supply (hypoxia) and appears clinically as an ischemic stroke, most usually due to thromboembolism. There are many different pathological processes that take place in infarcted brain tissue. These processes include energy depletion, membrane breakdown, inflammatory responses, excite-toxicity, mitochondrial dysfunction, tissue edema, necrosis, and cell death from a damaged area of blood brain barrier. In the end, the that tissue of brain is irreparably disrupted (Lee et al., 2000). In addition to its function as an antioxidant, hydrogen also plays a regulatory role in a number of signal transduction pathways as well as gene expression of many genes, some of which are involved in inflammatory processes. There is a lack of knowledge about molecular mechanisms that account for the numerous impacts that low concentrations of H2 have on signal transduction. As per the findings of a recent study, increasing oxidative stress may initiate oxidative chain dynamics, which then generate facilitators based on phospholipids. These mediators may have a role in changing signal transmission and gene expression (Iuchi et al., 2016). It has been hypothesised that molecular hydrogen, due to its neuroprotective properties, could be used as a medicine in treating brain disorders like dementia and brain damage caused by ischemia.
H2 Inhalation as Neuroprotective Agent in Different Studies and Clinical Trials:
Treatment with 2 percent quantity of hydrogen gas in between the reperfusion in ischemia or simply during the reperfusion lowered ischemia within one day but other behavioral deficits seven days later in the animal model of transient ischemic stroke (Ohsawa et al., 2007). In addition, spontaneously hypertensive rats with a predisposition to stroke who were given water with excessive hydrogen showed less infracts due to ischemia and stroke different areas of brain. It would appear that the mechanism behind this effect is the preservation of the blood-brain barrier and the inhibition of the matrix of enzymatic activity of metalloproteinase in some regions of the hippocampus (Takeuchi et al., 2015). After suffering global cerebral ischemia, mice treated with hydrogen had a seven-day survival rate that was 8.3 times higher than normal (Nagatani et al., 2012).
In a medical trial with 25 patients who had cerebral ischemia, the patients inhaled 3 percent hydrogen twice a day for an hour each for seven days. After 20 minutes, the amount of hydrogen in blood of arteries and veins blood topped out. After the delivery of hydrogen was stopped, he quantity of hydrogen in blood decreased upto 10% of plateau after 6–18 minutes, respectively. With the exception of an increase in oxygen gas saturation in the patients group of hydrogen, which showed clinical outcomes faster as compared to the control, vitals and regular blood tests remained unchanged. (Ono et al., 2012; Ono et al., 2017)
Research on cells, animals, and humans is accumulating more and more evidence that hydrogen can be administered in a risk-free manner as a neuro-protectant throughout the process of revascularization. The encouraging findings of the pilot research call for larger, randomized, studies to investigate the potential association of hydrogen gas in treating stroke.
Conclusion:
Numerous studies point to potential clinical outcomes hydrogen gas inhalation in the treatment, therapies, and preventions of a variety of neurological illnesses, despite the fact that these conditions are now untreatable in their entirety. According to our current understanding, there have been no incidents of adverse effects caused by H2, and its utilization in everyday medical practice is not only simple but also affordable and fruitful. However, more researches needs to be done to establish the appropriate dosage for each condition. In order to accomplish this goal, it will be necessary to elucidate the molecular mechanisms that are behind the biological repercussions of H2. It will pave way for the development of hydrogen gas as an efficient neuroprotective drug.
References:
· Emerit, J., Edeas, M. and Bricaire, F. (2004). Neurodegenerative diseases and oxidative stress. Biomedicine & Pharmacotherapy, [online] 58(1), pp.39–46. doi:10.1016/j.biopha.2003.11.004
· Ohsawa, I., Ishikawa, M., Takahashi, K., Watanabe, M., Nishimaki, K., Yamagata, K., Katsura, K., Katayama, Y., Asoh, S. and Ohta, S. (2007). Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nature Medicine, [online] 13(6), pp.688–694. doi:10.1038/nm1577
· Fukuda, K., Asoh, S., Ishikawa, M., Yamamoto, Y., Ohsawa, I. and Ohta, S. (2007). Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochemical and Biophysical Research Communications, [online] 361(3), pp.670–674. doi:10.1016/j.bbrc.2007.07.088.
· Hayashida, K., Sano, M., Ohsawa, I., Shinmura, K., Tamaki, K., Kimura, K., Endo, J., Katayama, T., Kawamura, A., Kohsaka, S., Makino, S., Ohta, S., Ogawa, S. and Fukuda, K. (2008). Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia–reperfusion injury. Biochemical and Biophysical Research Communications, [online] 373(1), pp.30–35. doi:10.1016/j.bbrc.2008.05.165
· Ohta, S. (2014). Molecular hydrogen as a preventive and therapeutic medical gas: initiation, development and potential of hydrogen medicine. Pharmacology & Therapeutics, [online] 144(1), pp.1–11. doi:10.1016/j.pharmthera.2014.04.006.
· Ichihara, M., Sobue, S., Ito, M., Ito, M., Hirayama, M. and Ohno, K. (2015). Beneficial biological effects and the underlying mechanisms of molecular hydrogen – comprehensive review of 321 original articles -. Medical Gas Research, [online] 5(1). doi:10.1186/s13618-015-0035-1.
· Itoh, T., Hamada, N., Terazawa, R., Ito, M., Ohno, K., Ichihara, M., Nozawa, Y. and Ito, M. (2011). Molecular hydrogen inhibits lipopolysaccharide/interferon γ-induced nitric oxide production through modulation of signal transduction in macrophages. Biochemical and Biophysical Research Communications, [online] 411(1), pp.143–149. doi:10.1016/j.bbrc.2011.06.116.
· Kajiya, M., Sato, K., Silva, M.J.B., Ouhara, K., Do, P.M., Shanmugam, K.T. and Kawai, T. (2009). Hydrogen from intestinal bacteria is protective for Concanavalin A-induced hepatitis. Biochemical and Biophysical Research Communications, [online] 386(2), pp.316–321. doi:10.1016/j.bbrc.2009.06.024
· Benjamin, E.J., Virani, S.S., Callaway, C.W., Chamberlain, A.M., Chang, A.R., Cheng, S., Chiuve, S.E., Cushman, M., Delling, F.N., Deo, R., de Ferranti, S.D., Ferguson, J.F., Fornage, M., Gillespie, C., Isasi, C.R., Jiménez, M.C., Jordan, L.C., Judd, S.E., Lackland, D. and Lichtman, J.H. (2018). Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association. Circulation, [online] 137(12). doi:10.1161/cir.0000000000000558
· Furie, K.L. and Jayaraman, M.V. (2018). 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke. Stroke, [online] 49(3), pp.509–510. doi:10.1161/strokeaha.118.020176
· Masumi Iketani and Ikuroh Ohsawa (2022). Molecular Hydrogen as a Neuroprotective Agent. Current Neuropharmacology, [online] 15(2), pp.324–331. Available at: https://benthamscience.com/article/76362
· Ichihara, M., Sobue, S., Ito, M., Ito, M., Hirayama, M. and Ohno, K. (2015). Beneficial biological effects and the underlying mechanisms of molecular hydrogen – comprehensive review of 321 original articles -. Medical Gas Research, [online] 5(1). doi:10.1186/s13618-015-0035-1
· Liu, Y., Liu, W., Sun, X., Li, R., Sun, Q., Cai, J., Kang, Z., Lv, S., Zhang, J.H. and Zhang, W. (2011). Hydrogen saline offers neuroprotection by reducing oxidative stress in a focal cerebral ischemia-reperfusion rat model. Medical Gas Research, [online] 1(1), p.15. doi:10.1186/2045-9912-1-15.
· Zhai, X., Chen, X., Shi, J., Shi, D., Ye, Z., Liu, W., Li, M., Wang, Q., Kang, Z., Bi, H. and Sun, X. (2013). Lactulose ameliorates cerebral ischemia–reperfusion injury in ratsby inducing hydrogen by activating Nrf2 expression. Free Radical Biology and Medicine, [online] 65, pp.731–741. doi:10.1016/j.freeradbiomed.2013.08.004
· Takeuchi, S., Nagatani, K., Otani, N., Nawashiro, H., Sugawara, T., Wada, K. and Mori, K. (2015). Hydrogen improves neurological function through attenuation of blood–brain barrier disruption in spontaneously hypertensive stroke-prone rats. BMC Neuroscience, [online] 16(1). doi:10.1186/s12868-015-0165-3
· Nagatani, K., Wada, K., Takeuchi, S., Kobayashi, H., Uozumi, Y., Otani, N., Fujita, M., Tachibana, S. and Nawashiro, H. (2012). Effect of Hydrogen Gas on the Survival Rate of Mice Following Global Cerebral Ischemia. Shock, [online] 37(6), pp.645–652. doi:10.1097/shk.0b013e31824ed57c.
· Ono, H., Nishijima, Y., Adachi, N., Sakamoto, M., Kudo, Y., Kaneko, K., Nakao, A. and Imaoka, T. (2012). A basic study on molecular hydrogen (H2) inhalation in acute cerebral ischemia patients for safety check with physiological parameters and measurement of blood H2 level. Medical Gas Research, [online] 2(1), p.21. doi:10.1186/2045-9912-2-21
· Ono, H., Nishijima, Y., Ohta, S., Sakamoto, M., Kinone, K., Horikosi, T., Tamaki, M., Takeshita, H., Futatuki, T., Ohishi, W., Ishiguro, T., Okamoto, S., Ishii, S. and Takanami, H. (2017). Hydrogen Gas Inhalation Treatment in Acute Cerebral Infarction: A Randomized Controlled Clinical Study on Safety and Neuroprotection. Journal of Stroke and Cerebrovascular Diseases, [online] 26(11), pp.2587–2594. doi:10.1016/j.jstrokecerebrovasdis.2017.06.012
· Lee, J.-M., Grabb, M.C., Zipfel, G.J. and Choi, D.W. (2000). Brain tissue responses to ischemia. Journal of Clinical Investigation, [online] 106(6), pp.723–731. doi:10.1172/jci11003.
· Iuchi, K., Imoto, A., Kamimura, N., Nishimaki, K., Ichimiya, H., Yokota, T. and Ohta, S. (2016). Molecular hydrogen regulates gene expression by modifying the free radical chain reaction-dependent generation of oxidized phospholipid mediators. Scientific Reports, [online] 6(1). doi:10.1038/srep18971.
· Ge, L., Yang, M., Yang, N.-N., Yin, X.-X. and Song, W.-G. (2017). Molecular hydrogen: a preventive and therapeutic medical gas for various diseases. Oncotarget, [online] 8(60), pp.102653–102673. doi:10.18632/oncotarget.21130.
· Hayashida, K., Sano, M., Ohsawa, I., Shinmura, K., Tamaki, K., Kimura, K., Endo, J., Katayama, T., Kawamura, A., Kohsaka, S., Makino, S., Ohta, S., Ogawa, S. and Fukuda, K. (2008). Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia–reperfusion injury. Biochemical and Biophysical Research Communications, [online] 373(1), pp.30–35. doi:10.1016/j.bbrc.2008.05.165.



