Molecular Hydrogen and its Role in Respiratory Diseases
November 18, 2022 2022-11-28 7:27Molecular Hydrogen and its Role in Respiratory Diseases

Molecular Hydrogen and its Role in Respiratory Diseases
Molecular Hydrogen and its Role in Respiratory Diseases
Introduction:
Hydrogen, an inert gas, has been utilised to help deep divers prevent oxygen deprivation. (Gardette and Delauze, 1996). It was discovered in 2007 that inhaled hydrogen had genuine protection against brain damage caused by ischemia and reperfusion because of its anti-inflammatory and anti-apoptotic properties (Fukuda et al., 2007). This report generated tremendous interest internationally. The anti-oxidation (Ji et al., 2010), anti-inflammatory (Kajiya et al., 2009), anti-apoptotic (Cai et al., 2008) characteristics of H2 have been examined relative to its therapeutic impact on a variety of disorders. Hydrogen in its diatomic form is quite a small molecule that dissipates rapidly in whole body as well as all the cells, and its impact is quite week that it doesn’t interfere with redox events of metabolism or the pathways of cell signaling. As a consequence of this, it has the potential to be an effective and risk-free antioxidant for respiratory illnesses. Inhalation, which is by far the most common method of drug administration, is regarded as the most effective approach to treating asthma. Ischemic heart disease (Hayashida et al., 2008) stroke (Cui et al., 2016), acute lung damage (Kohama et al., 2015) and inflammatory bowel illness (Kajiya et al., 2009) have been shown to benefit from or be protected through the H2 gas inhaling approach, as data has accumulated in recent years.
The lungs are the major organ that are responsible for exchanging gases with the rest of the circulatory system and the surrounding environment. They are continuously being exposed to a variety of potentially harmful external stimuli, including bacteria, viruses, and other germs, cigarette smoke, and airborne particulate matter, as they are breathing in and out. Injuries to the lungs, including respiratory and lung disorders, are frequently the result of either short-term or prolonged contact to these hazardous compounds.
Molecular Hydrogen- A Brief Introduction:
Hydrogen molecules are odorless, tasteless, and colorless, and their solubility in water is quite low. In mammalian cells, under the conditions of physiological activity, it is regarded to be non-active. Certain types of bacteria are capable, through the process of enzymatic catalysis, of extracting energy and electrons from molecular hydrogen. In addition, the anaerobic metabolism of bacteria results in the production of molecular hydrogen. Genes that code for enzymes containing iron or nickel that are required to speed up processes. These enzymes are necessary (Fritsch, Lenz and Friedrich, 2013). Molecular hydrogen, on the other hand, has just come to be seen as a unique physiologically appropriate molecule with significantly important applications. A group of scientists found that the selective quenching of hydroxyl free radical (OH) and per-oxy-nitrite ion is brought about by inhaling of 1-4% molecular hydrogen. This results in a considerable improvement in injury caused by oxidative stress produced by different ischemia of cerebral areas of brain.
(Fu and Zhang, 2022)
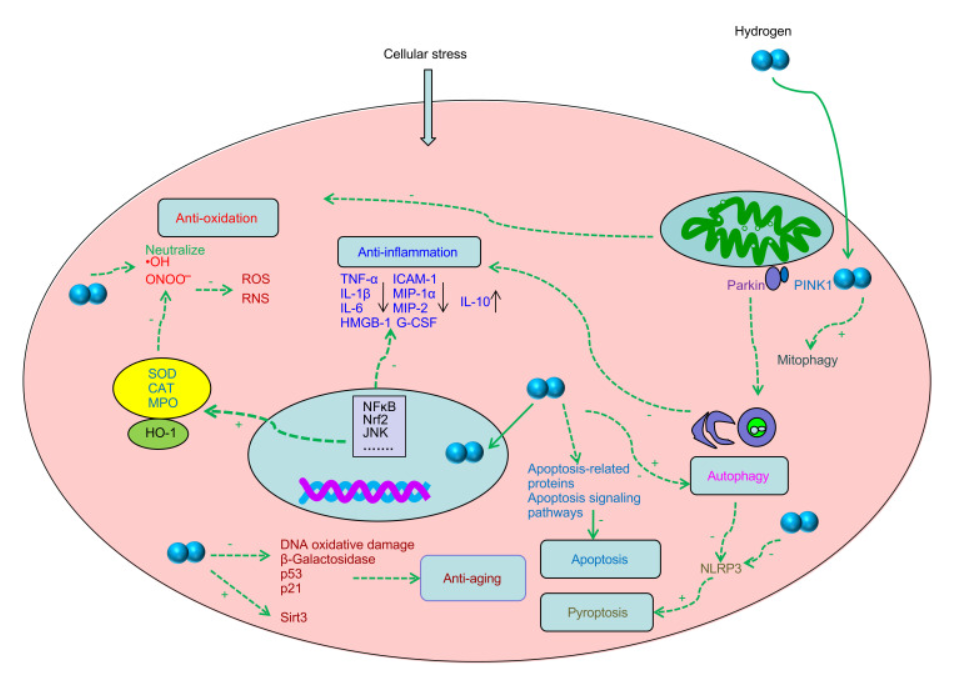
Figure.1 Diagram depicting the potential biological consequences of hydrogen.
Therapeutic Role of Molecular Hydrogen in Syndrome of Chronic Respiratory Dysfunction:
Severe failure in gas exchange due to a breakdown in the alveolar-capillary barrier and pulmonary edema characterizes both acute lung injury (ALI) and its more severe variant, acute respiratory distress syndrome (ARDS). Both of these conditions can be fatal. During the past two decades, advances in intensive care have led to enhance the percentage of patients with ARDS who survive, although the death rate is still quite increased (Goss et al., 2003; Maybauer et al., 2006). Many medications with properties of anti-inflammation are investigated as potential treatments for ALI. Palrnatine is able to alleviate lipo-polysaccharide-induced ALI in lab mice and it does this via inhibiting activated AKT pathway of cell signaling (Kan et al., 2021). Recent research conducted on animal models in laboratories has suggested that hydrogen may have the ability to mitigate the disease spread of (ALI) brought on by any factor that may include sepsis or LPS or even some pathogenic effects.
A therapy with hydrogen can lessen the severity of inflammatory response in the tissues of lung, suppress NF-kB activation-mediated inflammatory response and cell death, ultimately it significantly attenuates the recruitment of neutrophil blood cells that is brought on by LPS. The lower expression of MPO activity in lungs and pro-inflammatory chemokines is the mechanism behind these protective benefits (Xie et al., 2012). The expression and activation of p38MAPK are both greatly suppressed by hydrogen (Liang et al., 2012), as are levels of ROS that are p38MAPK-dependent (Shi et al., 2016). Hydrogen’s therapeutic potential for ALI induced by LPS is promising, and it may be even more so when combined with NO. This is possible despite hydrogen’s good efficacy when used alone. Nitro tyrosine, which is generated by the inhalation of NO by itself, is removed when this gas is combined with hydrogen, suggesting that the mechanism may include the interplay between two gaseous entities (Liu et al., 2015).
Hydrogen has the ability to attenuate the lung epithelial barrier dysfunction caused by LPS. Under typical circumstances, the alveolar epithelium functions as a substantial barrier that aids in the avoidance of edema of pulmonary regions.
Asthma and Molecular Hydrogen:
More over 300 million individuals all over the world are afflicted with asthma, making it one of the most prevalent non-infectious chronic diseases. (Vos et al., 2012; Khalaf et al., 2019). Asthma is defined by a variety of immunological pathways, and (Papi et al., 2018) it is the result of complicated interactions between genes and the environment, and it can manifest itself in a variety ways clinically. The allergic response of inflammation caused type-II increase the defense mechanisms at the surfaces that contain mucosa are considered to be the underlying cause of asthma (Kubo, 2017). This sort of response of immunity continues producing cytokines type-II (Kubo, 2017; Kim et al., 2010), such as different types of interleukins, as well as the switching majority of the antibodies to immunoglobulin E (Pulendran and Artis, 2012). The chronic and complex lung illness known as asthma is connected with inflammation of the airways, which in turn causes heightened sensitivity, restricted airflow, and altered airway structure (Haahtela, 1997; Comhair et al., 2000). Both internal and external reactive oxygen species play a significant part in the inflammation of airways and are factors that determine allergy in asthma severity (Comhair et al., 2001).
(Shastri et al., 2021)
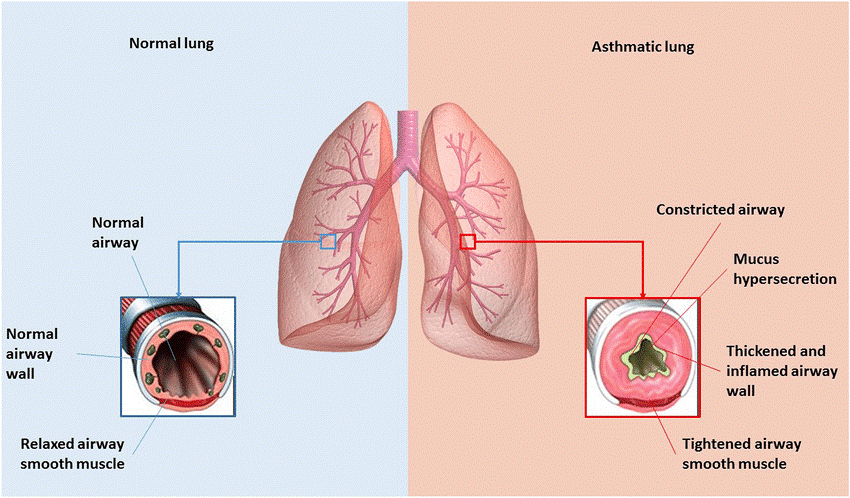
Figure.2 Comparing the normal lung to the asthmatic lung. Individuals in good health have normal airway walls and a relaxed airway smooth muscle. When asthmatic patients are exposed to harmless antigens, their airways constrict and become inflammatory with swelling walls and tightened smooth muscle.
In asthma, hydrogen showed to minimize both stress due to ROS and inflammation. In inflammation induced by ovalbumin, inhaling H2 gas decreases the amount of white blood cells in the bronchial-alveolar lavage fluid (BALF) especially eosinophils. It also significantly lowers the number of inflammation inducing pro-factors in the plasma, increases the catalysis of superoxide dismutase enzyme, and decreases in degree or intensity in the levels of certain genes like MPO and MDA (Zhang N et al., 2018). Inhibition of two activated pathways of NFkB and Nrf2, hydrogen significantly reduces airway hyper-responsiveness and goblet cell hyperplasia, as well as the response by T-helper-type-2, and it also reduces levels of IL-4 and immune-globin E (IgE). These effects can be attributed to hydrogen’s ability to alleviate goblet cell hyperplasia (Huang et al., 2019)
The role of macrophages of alveoli in lungs, under normal circumstances, is to keep a respiratory tract free of cell debri, pathogens like bacteria, and other foreign factors. The capability of macrophages of alveoli to phagocytose, on the other hand, is greatly diminished in asthma patients. Hydrogen gas, when inhaled, has the effect of correcting the incorrect phagocytosis that was occurring (Huang et al., 2019). Additionally, H2 gas inhibits the restructuring of airways, that leads to long-lasting changes in the structure & function of the airway wall. These changes, in turn, lead to a gradual decline in functioning of lungs (Fehrenbach., 2017). In the type of asthma induced by ovalbumin, HRS suppresses the pathway of NFkB, which causes a substantial reduction in mucus-index, MUC5-AC, and VEGF expression. Additionally, there is a reduction in the amount of deposition of collagen (Xiao et al., 2013). This significantly reduces the amount of modification that occurs in the passageways.
Based on the above mentioned findings, it appears that hydrogen gas reduces the inflammatory response due to allergy of the airways and enhances the proper functioning of airways in patients with asthma. These studies have helped us gain a better knowledge about advantages of molecular hydrogen gas as a medicinal and treatment intervention in asthma, despite the fact that the majority of research has been conducted on animal models, which only imperfectly replicate the symptoms of asthma. To determine the substantial benefits of hydrogen to be used in clinical usage, additional research is required.
Hydrogen’s impact on lung cancer:
Because of its great propensity for metastasis and its resistance to treatment, lung cancer has a dismal prognosis and is one of the most prevalent forms of cancer that results in death. Hydrogen proved to be quite effective against many malignancies, including different cancers especially lung cancer, in a growing number of studies including both laboratory animals and human participants. The following is a synopsis of the fundamental mechanisms that are responsible for hydrogen’s anti-cancer benefits.
The levels of protein expression that are responsible for angiogenesis and proliferation during cancer progression is downregulated by hydrogen, while protein expression that causes differentiation and development is upregulated by hydrogen (Liu et al., 2019). Moreover, H2 has the ability to lower the expression of regulator involved in condensation of chromatin known as structural maintenance of chromosome 3. It can also limit the invasive and migrating behavior of H195 and A549 cells, which stops the progression of lung cancer (Wang et al., 2018).
Apoptosis can be controlled by hydrogen. The abnormal regulation of apoptosis is one of the defining characteristics of cancer. Not only does disruption of apoptosis contribute to the genesis and progression of tumors, but it also plays a role in the resistance of tumors to treatment (Pistritto et al., 2016). Programmed cell death can be induced in lung tumor cells by hydrogen, and this is true for both types (Wang et al., 2018). Pathway of AKT/PI3K is a significant route that plays a role in both extracellular signaling events and cellular activities. These cellular functions include cell growth, apoptosis, and survival. Hydrogen, when paired with the PI3K inhibitor LY294002, was found to be effective in reducing cell proliferation and promoting apoptosis in non-small-cell-lung-cancer. This was accomplished by minimizing the phosphorylation of Akt protein and blocking the main pathway i.e. PI3K.
The treatment of more advanced stages of cancer responds favorably to hydrogen. Patients see a considerable improvement in their physical status as a result of this treatment, as well as a decrease in pain, anorexia, exhaustion, and increased amount of biomarkers specific to cancer. These effects are more noticeable among people who have been diagnosed with tumors of lung (Chen et al., 2019) Hydrogen therapy shows quite a lot of hope in the treatment of lung cancer because it is straightforward, inexpensive, and causes little adverse responses.
Conclusion:
There is emerging evidence indicating hydrogen’s extensive biological impacts. It controls apoptosis, inflammatory response, cellular ageing, and the process of autophagy. Numerous organs and systems have been shown to benefit from the protective and beneficial outcomes of H2 administration in animals in labs and human clinical trials. There is growing evidence that hydrogen has a preventive effect against a variety of lung illnesses. In addition to its beneficial outcomes directly on the lung tissues, H2 allows protection for tissues of lungs indirectly through its beneficial responses on other organs and tissues. Nevertheless, the specific chemical processes underpinning hydrogen benefits are still unknown, plus current knowledge of its response is primarily based on trials done on animals only. Human based clinical trials are still left to be examined. Consequently, a greater knowledge of the protective pathways that mediate the hydrogen efficacy might aid in developing targeted medicines for therapies of different pulmonary illnesses. Products of hydrogen are intriguing potential commodities for the treatment of a wide variety of respiratory illnesses on account of the numerous possible applications they could have, in addition to their safety, simplicity, and ease of use.
References:
· Bhattacharya, J. and Matthay, M.A. (2013) ‘Regulation and repair of the alveolar-capillary barrier in acute lung injury’, Annual Review of Physiology, 75, pp. 593–615. Available at: https://doi.org/10.1146/annurev-physiol-030212-183756.
· Cai, J. et al. (2008) ‘Hydrogen therapy reduces apoptosis in neonatal hypoxia-ischemia rat model’, Neuroscience Letters, 441(2), pp. 167–172. Available at: https://doi.org/10.1016/j.neulet.2008.05.077.
· Chen, J.-B. et al. (2019) ‘“Real world survey” of hydrogen-controlled cancer: a follow-up report of 82 advanced cancer patients’, Medical Gas Research, 9(3), pp. 115–121. Available at: https://doi.org/10.4103/2045-9912.266985.
· Comhair, S.A. et al. (2001) ‘Extracellular glutathione peroxidase induction in asthmatic lungs: evidence for redox regulation of expression in human airway epithelial cells’, FASEB journal: official publication of the Federation of American Societies for Experimental Biology, 15(1), pp. 70–78. Available at: https://doi.org/10.1096/fj.00-0085com.
· Cui, J. et al. (2016) ‘Inhalation of water electrolysis-derived hydrogen ameliorates cerebral ischemia-reperfusion injury in rats – A possible new hydrogen resource for clinical use’, Neuroscience, 335, pp. 232–241. Available at: https://doi.org/10.1016/j.neuroscience.2016.08.021.
· Fehrenbach, H., Wagner, C. and Wegmann, M. (2017) ‘Airway remodeling in asthma: what really matters’, Cell and Tissue Research, 367(3), pp. 551–569. Available at: https://doi.org/10.1007/s00441-016-2566-8.
· Fritsch, J., Lenz, O. and Friedrich, B. (2013) ‘Structure, function and biosynthesis of O₂-tolerant hydrogenases’, Nature Reviews. Microbiology, 11(2), pp. 106–114. Available at: https://doi.org/10.1038/nrmicro2940.
· Fukuda, K. et al. (2007) ‘Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress’, Biochemical and Biophysical Research Communications, 361(3), pp. 670–674. Available at: https://doi.org/10.1016/j.bbrc.2007.07.088.
· Gardette, B. and Delauze, H.G. (1996) ‘[Techniques of underwater intervention: means, methods, research and outlook]’, Bulletin De l’Academie Nationale De Medecine, 180(5), pp. 975–983.
· Goss, C.H. et al. (2003) ‘Incidence of acute lung injury in the United States’, Critical Care Medicine, 31(6), pp. 1607–1611. Available at: https://doi.org/10.1097/01.CCM.0000063475.65751.1D.
· Haahtela, T. (1997) ‘Airway remodelling takes place in asthma–what are the clinical implications?’, Clinical and Experimental Allergy: Journal of the British Society for Allergy and Clinical Immunology, 27(4), pp. 351–353.
· Hayashida, K. et al. (2008) ‘Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury’, Biochemical and Biophysical Research Communications, 373(1), pp. 30–35. Available at: https://doi.org/10.1016/j.bbrc.2008.05.165.
· Huang, P. et al. (2019) ‘Hydrogen gas inhalation enhances alveolar macrophage phagocytosis in an ovalbumin-induced asthma model’, International Immunopharmacology, 74, p. 105646. Available at: https://doi.org/10.1016/j.intimp.2019.05.031.
· Ji, X. et al. (2010) ‘Beneficial effects of hydrogen gas in a rat model of traumatic brain injury via reducing oxidative stress’, Brain Research, 1354, pp. 196–205. Available at: https://doi.org/10.1016/j.brainres.2010.07.038.
· Kajiya, M. et al. (2009) ‘Hydrogen mediates suppression of colon inflammation induced by dextran sodium sulfate’, Biochemical and Biophysical Research Communications, 386(1), pp. 11–15. Available at: https://doi.org/10.1016/j.bbrc.2009.05.117.
· Kan, X. et al. (2021) ‘Effect of Palrnatine on lipopolysaccharide-induced acute lung injury by inhibiting activation of the Akt/NF-κB pathway’, Journal of Zhejiang University. Science. B, 22(11), pp. 929–940. Available at: https://doi.org/10.1631/jzus.B2000583.
· Khalaf, K. et al. (2019) ‘Asthma from immune pathogenesis to precision medicine’, Seminars in Immunology, 46, p. 101294. Available at: https://doi.org/10.1016/j.smim.2019.101294.
· Kim, H.Y., DeKruyff, R.H. and Umetsu, D.T. (2010) ‘The many paths to asthma: phenotype shaped by innate and adaptive immunity’, Nature Immunology, 11(7), pp. 577–584. Available at: https://doi.org/10.1038/ni.1892.
· Kohama, K. et al. (2015) ‘Hydrogen inhalation protects against acute lung injury induced by hemorrhagic shock and resuscitation’, Surgery, 158(2), pp. 399–407. Available at: https://doi.org/10.1016/j.surg.2015.03.038.
· Kubo, M. (2017) ‘Innate and adaptive type 2 immunity in lung allergic inflammation’, Immunological Reviews, 278(1), pp. 162–172. Available at: https://doi.org/10.1111/imr.12557.
· Liang, C. et al. (2012) ‘[Effect of hydrogen inhalation on p38 MAPK activation in rats with lipopolysaccharide- induced acute lung injury]’, Nan Fang Yi Ke Da Xue Xue Bao = Journal of Southern Medical University, 32(8), pp. 1211–1213.
· Liu, H. et al. (2015) ‘Combination therapy with nitric oxide and molecular hydrogen in a murine model of acute lung injury’, Shock (Augusta, Ga.), 43(5), pp. 504–511. Available at: https://doi.org/10.1097/SHK.0000000000000316.
· Liu, M.-Y. et al. (2019) ‘Molecular hydrogen suppresses glioblastoma growth via inducing the glioma stem-like cell differentiation’, Stem Cell Research & Therapy, 10(1), p. 145. Available at: https://doi.org/10.1186/s13287-019-1241-x.
· Maybauer, M.O., Maybauer, D.M. and Herndon, D.N. (2006) ‘Incidence and outcomes of acute lung injury’, The New England Journal of Medicine, 354(4), pp. 416–417; author reply 416-417.
· Papi, A. et al. (2018) ‘Asthma’, The Lancet, 391(10122), pp. 783–800. Available at: https://doi.org/10.1016/S0140-6736(17)33311-1.
· Pistritto, G. et al. (2016) ‘Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies’, Aging, 8(4), pp. 603–619. Available at: https://doi.org/10.18632/aging.100934.
· Pulendran, B. and Artis, D. (2012) ‘New Paradigms in Type 2 Immunity’, Science, 337(6093), pp. 431–435. Available at: https://doi.org/10.1126/science.1221064.
· Shi, J., Gao, W. and Shao, F. (2017) ‘Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death’, Trends in Biochemical Sciences, 42(4), pp. 245–254. Available at: https://doi.org/10.1016/j.tibs.2016.10.004.
· Vos, T. et al. (2012) ‘Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010’, The Lancet, 380(9859), pp. 2163–2196. Available at: https://doi.org/10.1016/S0140-6736(12)61729-2.
· Wang, D. et al. (2018) ‘Hydrogen gas inhibits lung cancer progression through targeting SMC3’, Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 104, pp. 788–797. Available at: https://doi.org/10.1016/j.biopha.2018.05.055.
· Xiao, M. et al. (2013) ‘Hydrogen-rich saline reduces airway remodeling via inactivation of NF-κB in a murine model of asthma’, European Review for Medical and Pharmacological Sciences, 17(8), pp. 1033–1043.
· Xie, K. et al. (2012) ‘Molecular hydrogen ameliorates lipopolysaccharide-induced acute lung injury in mice through reducing inflammation and apoptosis’, Shock (Augusta, Ga.), 37(5), pp. 548–555. Available at: https://doi.org/10.1097/SHK.0b013e31824ddc81.
· Zhang, N. et al. (2018) ‘Inhalation of hydrogen gas attenuates airway inflammation and oxidative stress in allergic asthmatic mice’, Asthma Research and Practice, 4(1), p. 3. Available at: https://doi.org/10.1186/s40733-018-0040-y.
· Zhang, Y., Zhang, J. and Fu, Z. (2022) ‘Molecular hydrogen is a potential protective agent in the management of acute lung injury’, Molecular Medicine, 28(1), p. 27. Available at: https://doi.org/10.1186/s10020-022-00455-y.

